Structural Progression in Clusters of Ionized Water, (H2O)n=1–5+ | The Journal of Physical Chemistry A

Liquid-liquid equilibrium data for systems formed by PEG (4000 or 6000) or alcohol (1-propanol or 2-propanol) + potassium phosphate + water: Experimental measurements, correlations and thermodynamic modeling - ScienceDirect

Ion Hydration and Association in Aqueous Potassium Phosphate Solutions | The Journal of Physical Chemistry B

Synthetic Receptors with Micromolar Affinity for Chloride in Water - Sudan - Angewandte Chemie International Edition - Wiley Online Library

pH-Dependent Partitioning of Ionizable Organic Chemicals between the Silicone Polymer Polydimethylsiloxane (PDMS) and Water | ACS Environmental Au

Intramolecular hydroxyl nucleophilic attack pathway by a polymeric water oxidation catalyst with single cobalt sites | Nature Catalysis
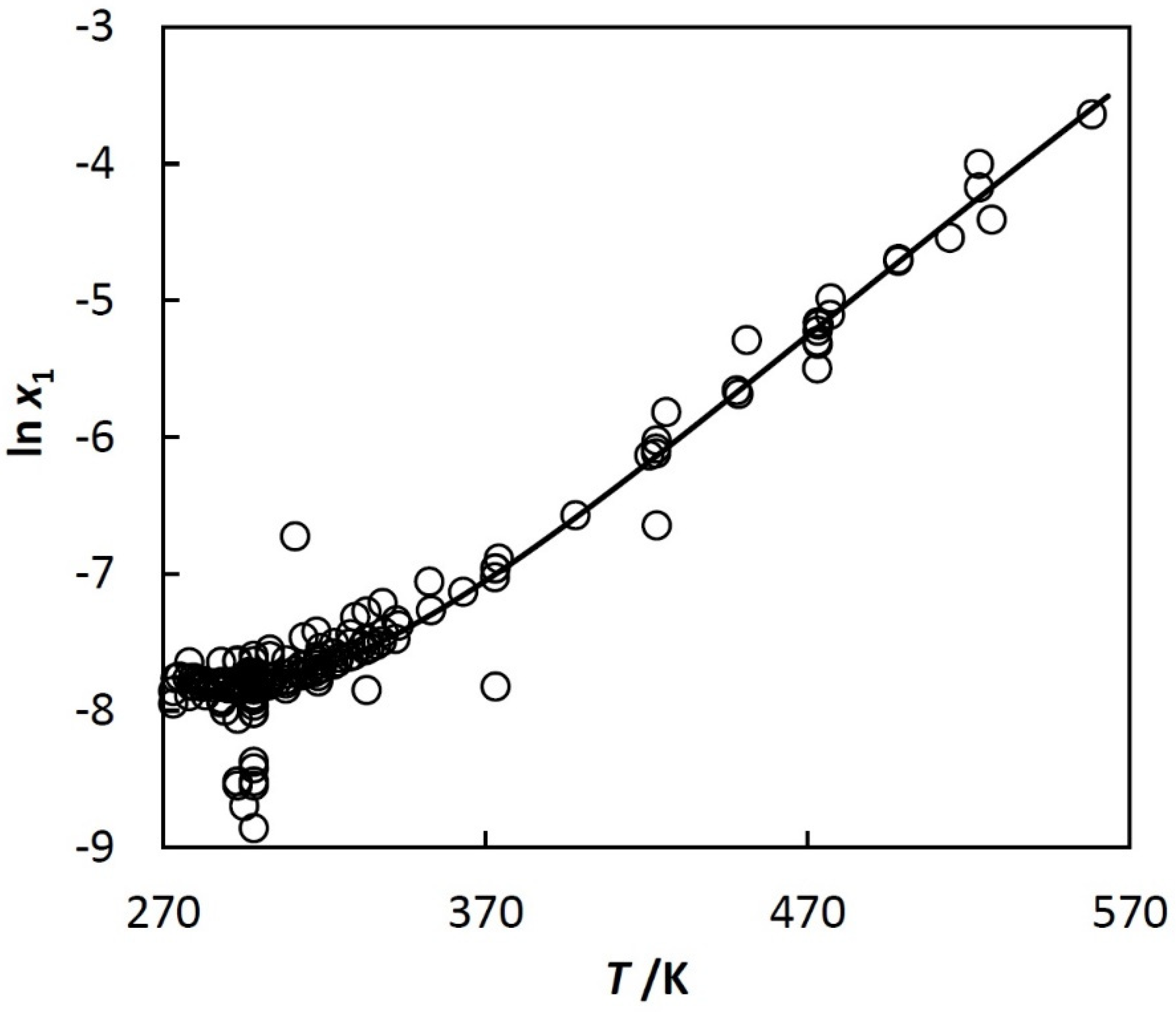
ChemEngineering | Free Full-Text | Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K

Cation Effects on Interfacial Water Organization of Aqueous Chloride Solutions. I. Monovalent Cations: Li+, Na+, K+, and NH4+ | The Journal of Physical Chemistry B

Phosphate sequestration by lanthanum-layered rare earth hydroxides through multiple mechanisms while avoiding the attenuation effect from sediment particles in lake water - ScienceDirect

Temperature Effect on the Phase Equilibrium of Polyethylene Glycol 2000 + Trilithium Citrate + Water Aqueous Two-Phase Systems at T = 288.15, 298.15, 308.15, and 318.15 K | Journal of Chemical & Engineering Data
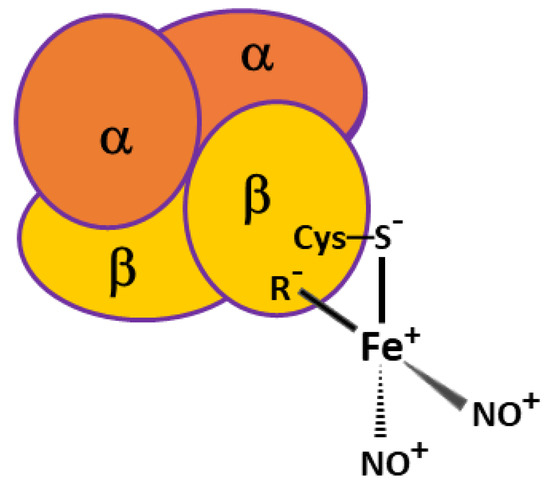
IJMS | Free Full-Text | Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite
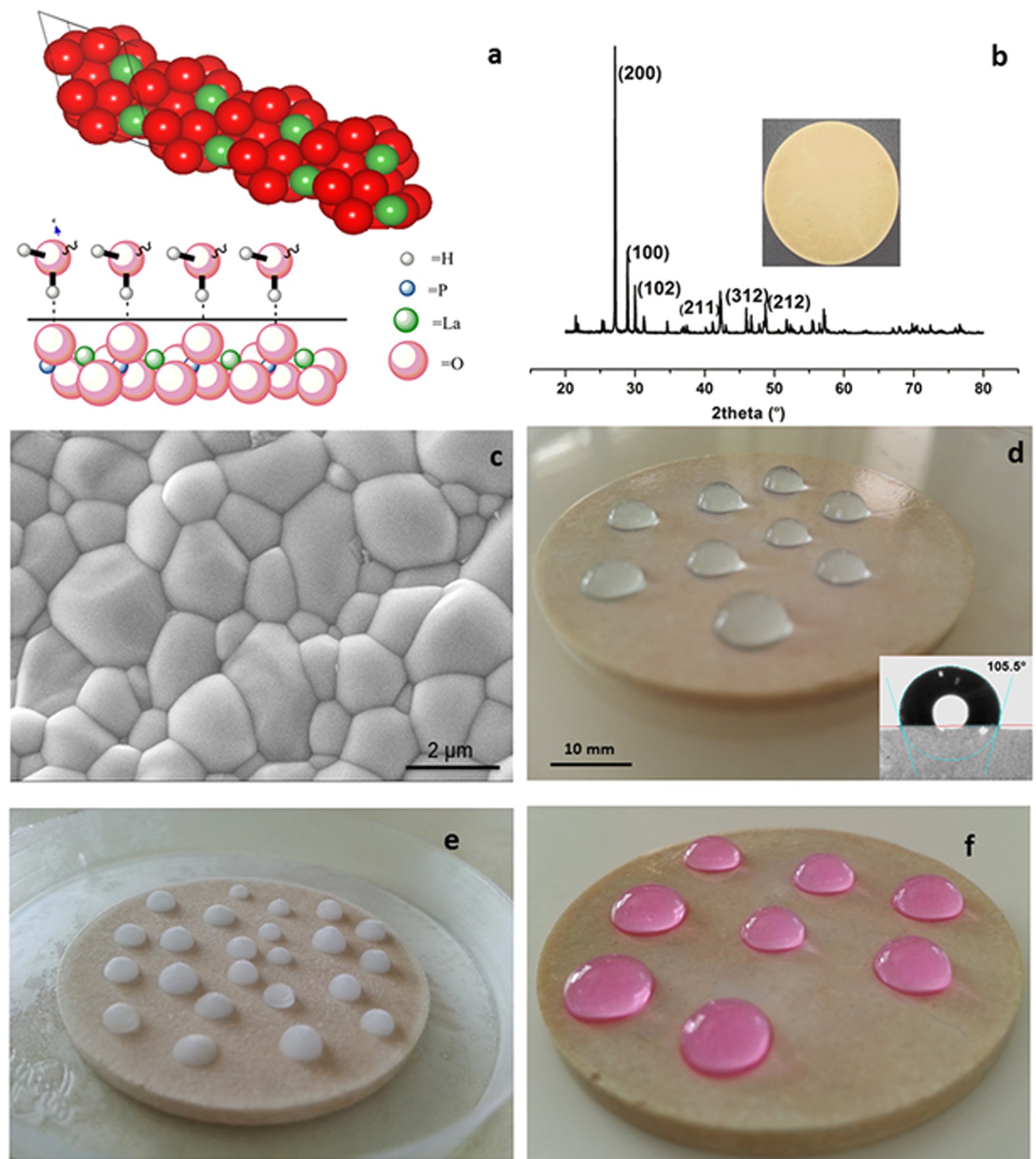
Hydrophobic and Metallophobic Surfaces: Highly Stable Non-wetting Inorganic Surfaces Based on Lanthanum Phosphate Nanorods | Scientific Reports

Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms | Environmental Science & Technology

Solid−liquid phase equilibrium for the ternary system (ammonium dihydrogen phosphate + agricultural grade ammonium polyphosphate (degree of polymerization ranged from 1 to 2) + water) at (278.2 and 313.2) K - ScienceDirect
Molecules | Free Full-Text | Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K)

Oxygen isotope exchange rates between phosphate and water catalyzed by inorganic pyrophosphatase: Implications for the biogeochemical cycle of phosphorus - ScienceDirect

General Principles and Strategies for Salting-Out Informed by the Hofmeister Series | Organic Process Research & Development
![Synthesis of the Hydroxide Cluster [Al13(μ3-OH)6(μ-OH)18(H2O)24]15+ from an Aqueous Solution | Inorganic Chemistry Synthesis of the Hydroxide Cluster [Al13(μ3-OH)6(μ-OH)18(H2O)24]15+ from an Aqueous Solution | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic200483q/asset/images/ic200483q.social.jpeg_v03)